

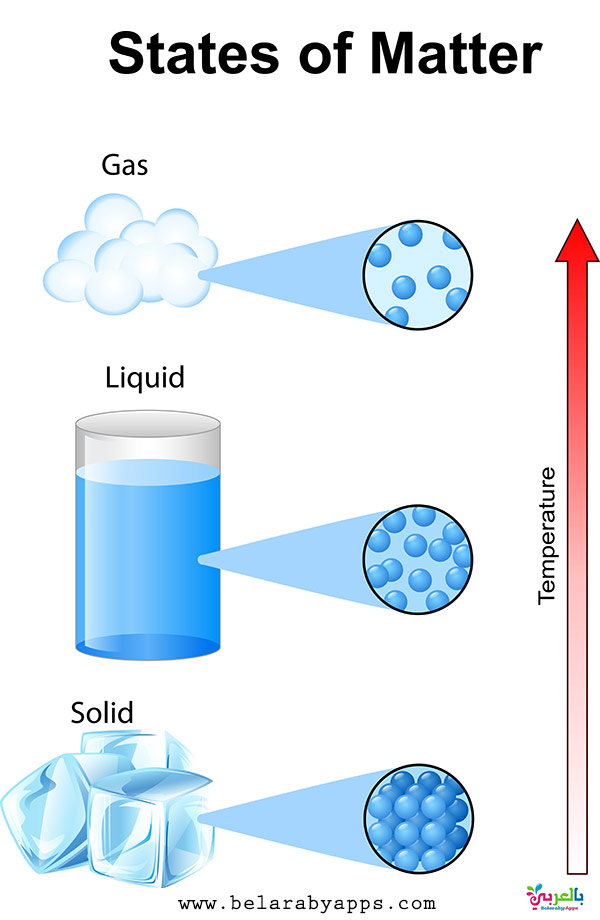
The diffusion rate in a solid-state of the matter is extremely low. In the gaseous state of matter, there is no intermolecular attraction between molecules. In the liquid state of matter, the force of attraction between molecules is quite weak. In the solid form of matter, the force of attraction between molecules is extremely strong. The intermolecular space is free-flowing and abundant in the gaseous form of matter. In the liquid state of matter, the intermolecular space is moderate, but it exists.
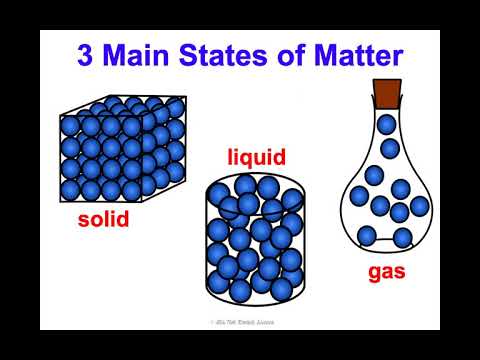
Molecules are grouped sparsely at random in the gaseous state of matter. In the liquid state of matter, molecules are grouped sparsely at random. Molecules are organized in a regular and close pattern in the solid-state of matter. The gaseous state of matter neither has fixed volume nor shape. The liquid state of matter does not have a defined shape, they tend to shape according to the container. Solid-state of matter has fixed volume and shape. Gas is a state of matter that does not have a shape and instead takes on the shape of the container it is placed in. They are fluids with no fixed structure but a defined volume. It's a type of matter with a solid structure and a distinct shape.


 0 kommentar(er)
0 kommentar(er)
